TL;DR:
- BESSY II’s high-brilliance X-rays enable high-resolution microscopic imaging of cell organelles.
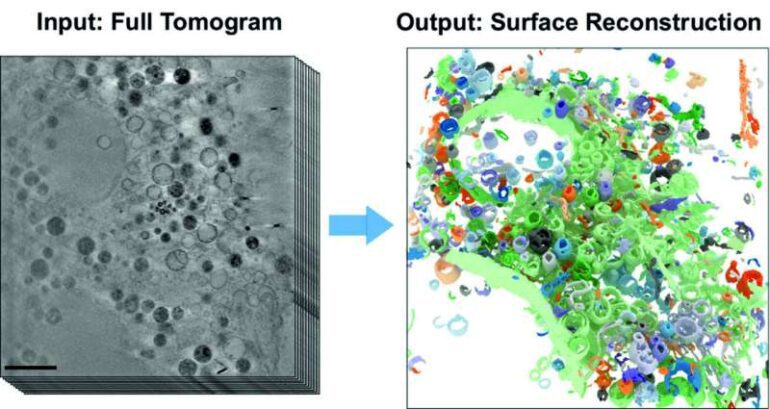
- Cryo X-ray tomography provides detailed 3D images of cell structures, which is ideal for studying changes caused by external triggers.
- Manual analysis of 3D tomograms is labor-intensive, prompting the development of a self-learning AI algorithm.
- The algorithm automates the detection of subcellular structures, accelerating quantitative analysis of 3D X-ray data sets.
- The AI-based analysis method achieves a recognition accuracy of approximately 70% and enables faster evaluation of cell features.
- Cryo X-ray microscopy allows for the study of cells in their natural state by shock-freezing samples.
- This new analysis method holds the potential for investigating cell responses to environmental influences more rapidly and reliably.
- The fusion of AI and X-ray microscopy opens up new possibilities for uncovering cellular dynamics and interactions.
Main AI News:
Advancements in the field of cell biology have always relied on cutting-edge technologies to unlock the mysteries hidden within the microscopic world. Now, with the aid of artificial intelligence (AI) and high-brilliance X-rays, researchers are pushing the boundaries of analysis even further. BESSY II, a renowned facility, has harnessed the power of X-ray microscopy to delve into the intricate world of cell organelles with unparalleled precision.
In the past, studying the three-dimensional structures of cell organelles required arduous manual labor and painstaking data analysis. However, a groundbreaking collaboration between Prof. Dr. Frank Noé, a distinguished computer scientist, and Prof. Dr. Helge Ewers, an esteemed cell biologist, both from Freie Universität Berlin, has paved the way for a transformative solution. Together with the X-ray microscopy department at HZB, they have developed a cutting-edge, self-learning algorithm that revolutionizes the quantitative analysis of 3D X-ray data sets.
The key to this innovation lies in the automated detection of subcellular structures, made possible by the powerful AI-based analysis method. By leveraging the capabilities of the newly developed algorithm, researchers can accelerate the evaluation process, saving invaluable time and resources. Utilizing the U41 beamline at BESSY II, scientists have acquired remarkable 3D images of the intricate interior of biological samples.
Dr. Stephan Werner, an expert in X-ray microscopy at HZB, highlights the significance of this achievement: “In this study, we have successfully demonstrated the effectiveness of AI-based analysis in studying cell volumes. We specifically focused on mammalian cells derived from cell cultures featuring filopodia, which are vital for cell migration.” Mammalian cells are known for their complex structures, housing various organelles, each dedicated to distinct cellular functions. The clarity and detail provided by cryo X-ray tomography enable researchers to observe changes in cell structures caused by external triggers.
The process begins by rapidly shock-freezing the cell samples, preventing the formation of ice crystals within the delicate structures. This ensures that the cells retain their natural state, allowing for a comprehensive study of the structural influence exerted by external factors. Such advancements in cryo X-ray microscopy have attracted considerable attention, as experts recognize the immense potential for accelerated and reliable investigations into how cells react to environmental influences, such as nanoparticles, viruses, or carcinogens.
Michael Dyhr, the first author of this groundbreaking work from Freie Universität Berlin, expresses enthusiasm about the promising future prospects: “Our work has already garnered significant interest among experts. The neural network embedded within the algorithm demonstrates an impressive accuracy of approximately 70% in identifying various cell features within a remarkably short timeframe. This novel analysis method holds the key to swiftly and reliably studying cellular responses to environmental factors, surpassing the limitations of conventional approaches.”
Conclusion:
The integration of artificial intelligence into the quantitative analysis of cell organelles through cryo X-ray microscopy represents a significant breakthrough. The automated detection and accelerated evaluation of subcellular structures offer researchers a more efficient means of studying cell features and their response to environmental influences. This innovation has the potential to revolutionize the field of cell biology, enabling faster and more reliable investigations. The market implications are vast, as this advancement empowers scientists to delve deeper into the intricacies of cellular dynamics, paving the way for new discoveries, breakthrough therapies, and enhanced understanding of biological systems. As a result, companies involved in microscopy technologies and AI-driven analytical tools will likely experience increased demand and growth opportunities in the scientific research and healthcare sectors.