TL;DR:
- RapidAI, a Silicon Valley-based AI startup, raises $75 million in a series C funding round.
- The funds will be used to expand their AI software tools for detecting strokes, aneurysms, and other neurological conditions in brain CT scans.
- The company’s recent FDA-cleared tools include Rapid NCCT Stroke and Rapid RV/LV, enhancing diagnostic precision for critical brain conditions.
- RapidAI’s machine learning AI software can identify cerebral aneurysms, analyze CT perfusion, and assess stroke severity using the Alberta Stroke Program Early CT Score system.
- The platform has analyzed over 10 million scans and is widely used in thousands of hospitals across 100 countries.
Main AI News:
RapidAI, the trailblazing AI-driven startup, has set a new precedent with its recent $75 million series C funding round, catapulting its ambitious mission to detect early signs of strokes, aneurysms, and other neurological conditions in brain CT scans to even greater heights. This Silicon Valley-based company is poised for rapid expansion, leveraging this substantial funding infusion not only to bolster the reach of its existing AI software tools but also to spearhead groundbreaking initiatives in previously uncharted disease areas, according to a company announcement that reverberated throughout the industry.
The funding milestone comes as a testament to RapidAI’s unwavering commitment to driving innovation and excellence in the realm of medical technology. A mere three years after securing $25 million in a series B funding round, with Lennertz & Co. leading the charge in September 2020, RapidAI continues to garner the attention of esteemed investors. This time, the baton was passed to Vista Credit Partners, whose resounding confidence in RapidAI’s potential is matched only by its financial backing. Notably, Vista Credit Partners has pledged to provide “operational support,” fortifying RapidAI’s growth trajectory under the watchful guidance of David Flannery, the investor’s president.
Under the astute leadership of CEO Karim Karti, RapidAI has cemented its position as a stalwart in the medical technology landscape over the past decade, initially establishing itself under the name iSchemaView. The company boasts a portfolio of FDA-cleared tools that autonomously analyze CT scans, unveiling early warning signs of critical brain conditions, safeguarding lives in the process.
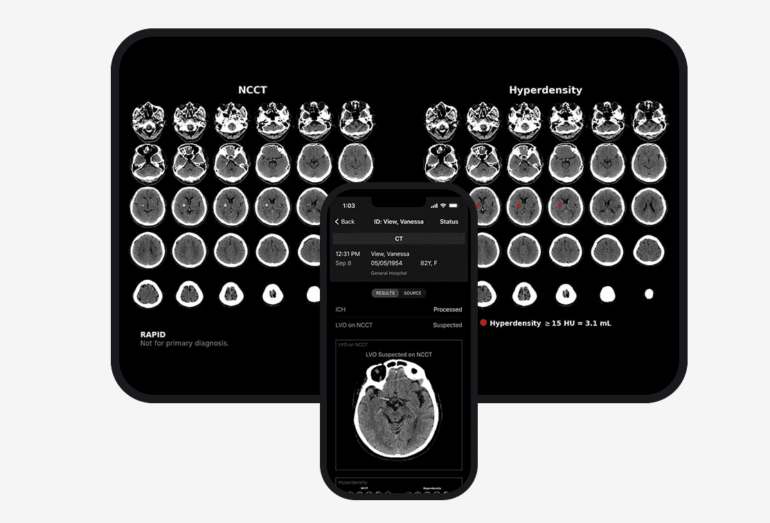
One of RapidAI’s recent triumphs is the Rapid NCCT Stroke technology—a marvel of innovation—cleared by the FDA earlier this year. This cutting-edge tool discerns possible cases of intracranial hemorrhage and large vessel occlusion from non-contrast CT images, significantly expediting diagnostic precision. Hot on the heels of this achievement, RapidAI secured regulatory approval for Rapid RV/LV, an add-on to their renowned Rapid PE solution for pulmonary embolism detection. This remarkable addition calculates the ratio between the right and left ventricles, providing crucial insights into the severity of suspected embolism cases.
Unyielding in its pursuit of excellence, RapidAI has also unveiled machine learning AI software capable of detecting cerebral aneurysms, analyzing CT perfusion, evaluating stroke severity using the Alberta Stroke Program Early CT Score system, and identifying cases of hyperdensity—critical signs of intracerebral hemorrhage.
With an unparalleled track record, RapidAI’s platform has already analyzed over 10 million scans and garnered widespread adoption across thousands of hospitals in over 100 countries. As hospitals seek solutions to address pressing needs, RapidAI emerges as the premier provider of advanced clinical decision support software. Going beyond mere triage and notification, RapidAI’s software delivers precise, contextually relevant data with unparalleled accuracy, empowering care teams and revolutionizing patient outcomes in the realm of critical healthcare.
Conclusion:
RapidAI’s substantial funding infusion and technological advancements in clinical AI solutions mark a significant milestone for the market. With an ever-growing portfolio of FDA-cleared tools and widespread adoption in thousands of hospitals worldwide, RapidAI’s cutting-edge software is poised to revolutionize neurological condition detection, drive improved patient outcomes, and solidify its leadership position in the medical technology landscape. As the company continues to innovate and expand, the market can expect transformative advancements in AI-driven healthcare solutions, paving the way for more efficient and accurate diagnosis and treatment of life-threatening neurological diseases.