TL;DR:
- MRI scans excel in soft tissue contrast, but motion can lead to image artifacts.
- MIT researchers combine deep learning and physics to correct motion-distorted MRI scans.
- Their model generates motion-free images while maintaining the scanning process.
- The method ensures consistency between images and real measurements, improving diagnostic accuracy.
- Neurological disorders like Alzheimer’s could benefit from motion-free MRI.
- Motion-related issues affect 15% of brain MRI scans, leading to increased hospital costs.
- Future research may address complex motion patterns in various body regions.
Main AI News:
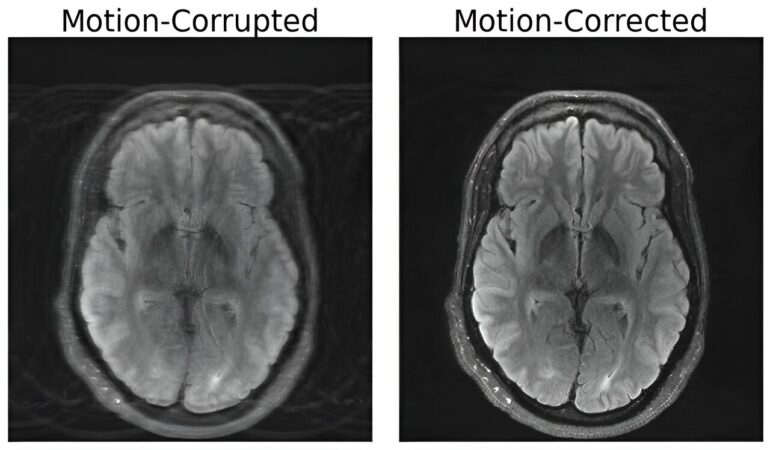
In the realm of medical imaging, Magnetic Resonance Imaging (MRI) has stood out for its unparalleled ability to provide high-resolution soft tissue contrast. However, the Achilles’ heel of this technology lies in its susceptibility to motion artifacts, often caused by the slightest patient movement during scans. Unlike X-rays or CT scans, where motion-induced blur is localized, MRI’s motion disruptions can corrupt entire images, potentially leading to misdiagnoses and compromised treatment decisions. Addressing this challenge head-on, a team of researchers at MIT has ingeniously merged deep learning with physics to develop a groundbreaking model that restores integrity to motion-corrupted brain MRI scans.
Nalini Singh, a distinguished Ph.D. candidate affiliated with the Abdul Latif Jameel Clinic for Machine Learning in Health at Harvard-MIT Program in Health Sciences and Technology, spearheads this remarkable endeavor. Singh, the lead author of the paper titled “Data Consistent Deep Rigid MRI Motion Correction,” underscores the ubiquity of motion-related issues in MRI scans and the relatively protracted nature of MRI imaging sessions.
The durations of MRI scans span a wide range, varying from several minutes to an hour, contingent upon the complexity of the images sought. In this timeframe, even minuscule movements exert a significant impact on image quality. Unlike traditional camera imaging, where motion effects often manifest as localized blurriness, MRI’s disruptions manifest as pervasive artifacts that compromise the entire image’s fidelity. Attempts to mitigate this challenge have included instructing patients to minimize motion by, for example, curbing deep breaths or even inducing anesthesia. However, these strategies are impractical for certain demographics, such as children or patients afflicted with psychiatric conditions.
The crux of the solution devised by the MIT team lies in the seamless fusion of physics-based modeling and advanced deep learning techniques. Their approach entails generating pristine, motion-free images from the very same motion-distorted data, all the while preserving the essence of the scanning procedure. “Our aim was to combine physics-based modeling and deep learning to get the best of both worlds,” Singh elucidates.
A pivotal facet of this innovative fusion revolves around upholding congruence between the resultant images and the genuine measurements of the depicted subject. This safeguard ensures that the model doesn’t generate “hallucinatory” images—superficially realistic yet inherently distorted representations—thereby averting the risk of exacerbating diagnostic accuracy.
Beyond individual well-being, the implications of this harmonious convergence extend far and wide. Neurological disorders, such as Alzheimer’s or Parkinson’s disease, which entail involuntary movements, can significantly benefit from motion-free MRI scans. An assessment from the University of Washington Department of Radiology suggests that around 15 percent of brain MRI scans are hampered by motion artifacts. When such disruptions necessitate additional scans to achieve diagnostically sufficient quality, hospitals incur substantial annual expenses, totaling around $115,000 per scanner.
While the current achievement is monumental, Singh envisions a roadmap ahead. Future undertakings may delve into more intricate categories of motion, encompassing diverse regions of the body. A prime example is fetal MRI, fraught with rapid and unpredictable motion that cannot be solely addressed through elementary translations and rotations.
Daniel Moyer, an esteemed assistant professor at Vanderbilt University, attests to the transformative potential of this breakthrough. “This line of work from Singh and company is the next step in MRI motion correction. Not only is it excellent research work, but I believe these methods will be used in all kinds of clinical cases: children and older folks who can’t sit still in the scanner, pathologies that induce motion, studies of moving tissue, even healthy patients will move in the magnet,” Moyer affirms. He further envisions that this research’s legacy will extend into routine clinical practice, fundamentally altering how medical images are processed and interpreted.
Conclusion:
The integration of deep learning and physics to mitigate motion artifacts in MRI scans represents a paradigm shift in medical imaging. This innovation addresses a longstanding challenge, enhancing the accuracy of diagnoses and potentially revolutionizing the clinical application of MRI technology. As a result, the market for medical imaging solutions could witness transformative growth, catering to a broader spectrum of patients and significantly reducing operational costs for healthcare providers.