TL;DR:
- Brainomix, the AI-powered medtech solutions company, launches its Brainomix 360 platform in the US.
- The platform includes FDA-cleared modules, such as e-ASPECTS, to support stroke care decisions.
- Brainomix aims to expand its transformative technology to more US stroke centers.
- The Brainomix 360 platform utilizes state-of-the-art AI algorithms for real-time brain scan interpretation.
- Dr. Waleed Brinjikji from the Mayo Clinic endorses the platform’s accuracy and potential.
- Brainomix secures recent FDA clearances for e-CTP and e-MRI modules, aiding thrombolysis and thrombectomy decisions.
- New notification tools, Triage LVO and Triage ICH, assist in identifying critical conditions.
- Brainomix establishes a strong US presence, with a focus on nationwide hospital network expansion.
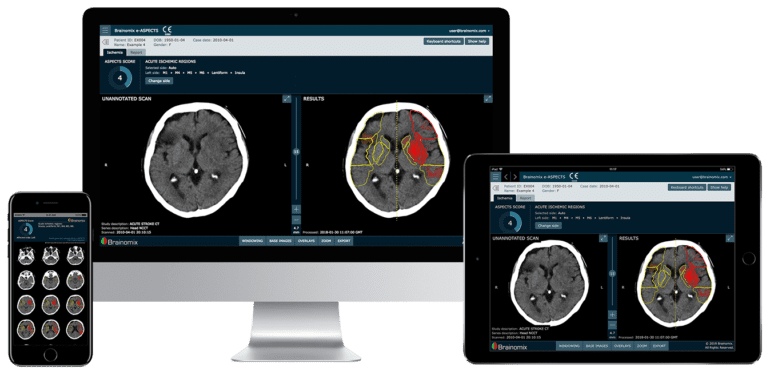
- The e-ASPECTS module, powered by explainable AI, enhances diagnostic precision.
- Brainomix’s legacy of scientific excellence and success in Europe drives its US market entry.
- The AI stroke software has been validated in over 60 publications, improving patient outcomes and treatment rates.
Main AI News:
In a strategic move aimed at transforming stroke care in the United States, Brainomix, the pioneering AI-powered medtech solutions company, has launched its comprehensive Brainomix 360 platform. This milestone follows a series of FDA clearances, including the highly anticipated e-ASPECTS module, and represents a significant step forward in the company’s mission to support clinicians in making informed imaging-based treatment decisions across the entire stroke pathway.
With a strong presence and a track record of market leadership in Europe, Brainomix is poised to introduce its groundbreaking technology to a wider audience of US stroke centers. At the core of the Brainomix 360 platform are cutting-edge AI algorithms that deliver real-time interpretation of brain scans, facilitating crucial treatment and transfer decisions for stroke patients. The ultimate goal is to ensure that every patient receives the right treatment at the right place and at the right time.
The launch of Brainomix’s US expansion was marked by its participation in the Society of Vascular and Interventional Neurology (SVIN) Conference in Miami, where Dr. Waleed Brinjikji, a distinguished expert in Radiology and Neurosurgery at the Mayo Clinic in Rochester, Minnesota, delivered a keynote presentation sharing his firsthand experience with the Brainomix 360 platform.
Dr. Brinjikji commented, “We have been collaborating with the Brainomix team on numerous research projects over the past couple of years, including a recent study that validated the performance of their e-ASPECTS module. The results demonstrated a significant improvement in ASPECTS scoring accuracy across different medical disciplines and levels of experience, making the e-ASPECTS module an invaluable tool for clinicians managing stroke patients across the US.”
Dr. Michalis Papadakis, co-founder and CEO of Brainomix, expressed his enthusiasm for the US launch, stating, “We are delighted to have the opportunity to introduce our Brainomix 360 platform to an ever-expanding network of US stroke centers and to showcase the extensive validation of our technology. Much of this validation has been conducted in the US, with institutions such as the Mayo Clinic, Emory University, Mount Sinai in New York, and UCLA contributing to our success.”
The recent FDA clearances include Brainomix 360 e-CTP and Brainomix 360 e-MRI, software modules that offer critical support for thrombolysis and thrombectomy treatment decisions, especially for late-window patients presenting to hospitals more than 6-12 hours after the onset of a stroke. Additionally, Brainomix 360 Triage LVO and Brainomix 360 Triage ICH are two innovative notification tools that provide real-time alerts to clinicians when a bleed or large vessel occlusion (LVO) is suspected, aiding in the assessment of a patient’s eligibility for mechanical thrombectomy.
Brainomix has already established a strong commercial presence in the United States and plans to expand its reach further by making its products available to hospital networks across the country. The company’s flagship software, the e-ASPECTS module, is powered by patented, explainable AI and assesses non-contrast CT scans to generate an ASPECTS score automatically. It also features a unique overlaid heatmap that enables a more nuanced assessment of each region, enhancing the precision of diagnostic evaluations.
Dr. Papadakis emphasized Brainomix’s rich heritage of scientific and academic excellence, originating as a spin-out from the University of Oxford. This legacy has paved the way for the company’s remarkable success in the UK and Europe, with national-level deployments of Brainomix 360 in Hungary and Wales, along with extensive roll-outs in England, Poland, Sweden, Italy, and Spain.
With deployments spanning more than 30 countries, Brainomix’s AI stroke software has been the subject of over 60 publications, highlighting its efficacy and impact on stroke care. Recent studies have shown that the implementation of Brainomix software significantly reduces door-in-door-out times by over an hour, leading to improved patient outcomes and a threefold increase in the number of patients achieving functional independence after stroke. Moreover, the software has contributed to a more than 50% increase in the rates of both thrombolysis and thrombectomy, underscoring its pivotal role in revolutionizing stroke care worldwide.
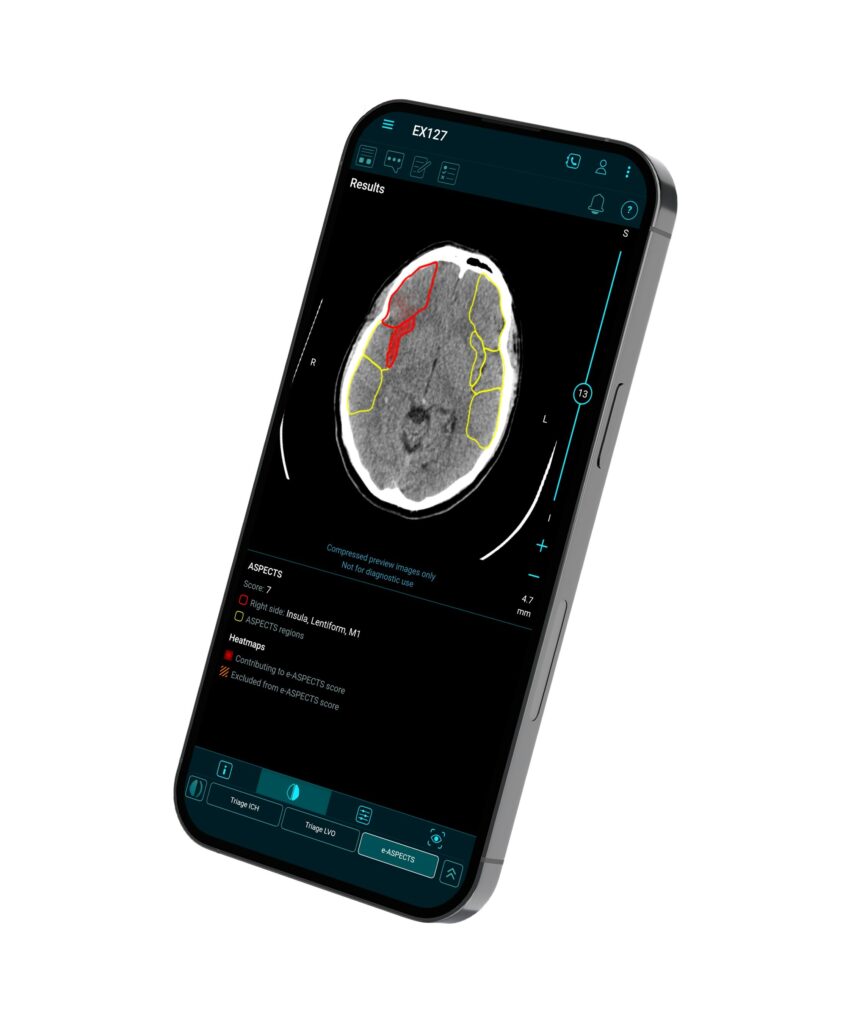
Brainomix 360 e-ASPECTS on a smartphone – an FDA-cleared decision support tool for assessing stroke signs on plain CT brain scans. Source: Cision US Inc.
Conclusion:
Brainomix’s entry into the US market with its FDA-cleared Brainomix 360 platform signifies a significant advancement in stroke care. The platform’s innovative AI algorithms and modules promise to enhance diagnostic accuracy, reduce treatment delays, and improve patient outcomes. This move is poised to disrupt the stroke care market by offering clinicians a powerful tool to make informed decisions, ultimately benefiting stroke patients across the United States.