TL;DR:
- Researchers introduce a pioneering physics-informed deep learning model.
- Focus on precise prediction of intratumoral fluid pressure (IFP) and liposome accumulation.
- Enhanced permeability and retention (EPR) effect’s role in drug delivery is highlighted.
- Physics-informed machine learning applied to synthetic tumor data for accurate predictions.
- Potential for personalized cancer treatments and enhanced therapeutic interventions.
Main AI News:
In the ever-evolving landscape of cancer therapies, a paradigm-shifting solution has emerged, one that promises to revolutionize our understanding of tumor dynamics. This groundbreaking study centers its focus on the precise prediction of intratumoral fluid pressure (IFP) and the accumulation of liposomes, introducing a pioneering physics-informed deep learning model. This innovative approach carries immense potential in optimizing cancer treatment strategies, offering precise insights into the distribution of therapeutic agents within tumors.
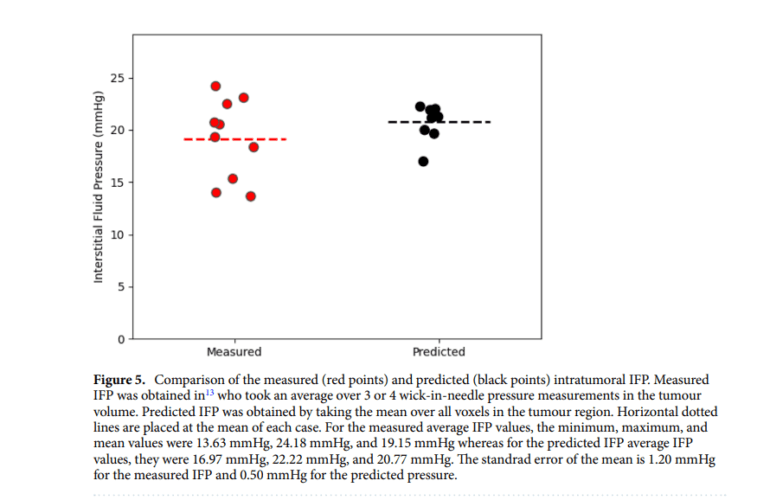
At the heart of many nanotherapeutics lies the enhanced permeability and retention (EPR) effect, capitalizing on distinct tumor attributes like heightened vascular permeability and transvascular pressure gradients. However, the impact of the EPR effect on treatment outcomes has exhibited inconsistency, prompting an in-depth exploration of the factors influencing drug delivery within solid tumors. Among these factors, interstitial fluid pressure (IFP) has emerged as a pivotal determinant, severely constraining the delivery of liposomal drugs to the central regions of tumors. Furthermore, elevated IFP stands as an independent prognostic marker, exerting a significant influence on the efficacy of radiation therapy and chemotherapy for specific solid cancers.
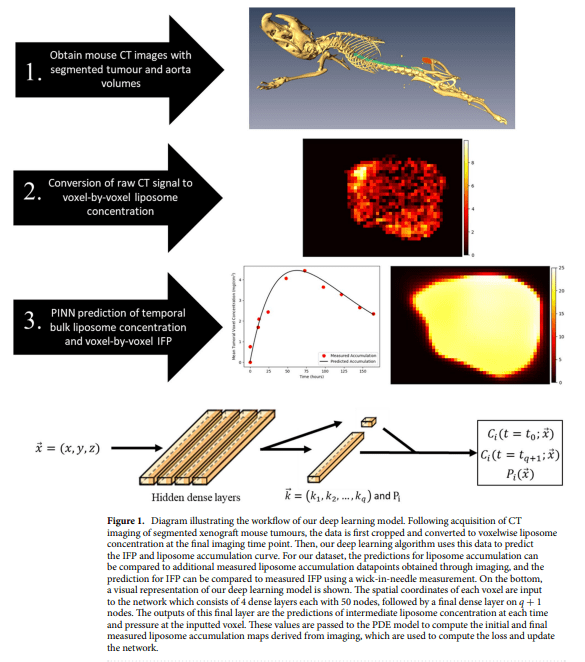
To confront these challenges head-on, researchers present an advanced model that predicts voxel-by-voxel intratumoral liposome accumulation and IFP utilizing pre and post-administration imaging data. What sets their approach apart is the integration of physics-informed machine learning, a cutting-edge fusion of machine learning with partial differential equations. By applying this innovative technique to a dataset generated synthetically, the researchers demonstrate the model’s capacity to deliver highly accurate predictions with minimal input data.
Traditional methodologies often struggle to provide consistent and precise predictions of liposome distribution and IFP within tumors. This research’s contribution distinguishes itself by introducing an unprecedented approach that amalgamates machine learning with principles grounded in physics. This pioneering model not only assures precise predictions but also carries immediate implications for the design of cancer treatments. The ability to anticipate the spatial distribution of liposomes and IFP within tumors opens up new horizons for a deeper comprehension of tumor dynamics, paving the way for more effective and personalized therapeutic interventions.
Delving into the intricacies of their proposed method, a team of researchers from the University of Waterloo and the University of Washington elucidates the application of physics-informed deep learning to achieve predictions at the voxel level. The model’s reliance on synthetic tumor data underscores its resilience and efficiency, offering a potential remedy to the challenges posed by elevated IFP in cancer treatment. By showcasing the scalability and applicability of their approach with minimal input data, the researchers underscore its potential in predicting tumor progression and streamlining treatment planning.
Source: Marktechpost Media Inc.
Conclusion:
The introduction of a physics-informed deep learning model for predicting IFP and liposome accumulation in tumors signifies a groundbreaking development in cancer treatment. This innovation holds the promise of optimizing treatment strategies and enhancing therapeutic interventions, potentially reshaping the market by offering more personalized and effective cancer treatments.