TL;DR:
- Formus Labs, based in New Zealand, obtains FDA 510(k) clearance for its hip surgery planning software.
- The software, called Formus Hip, utilizes AI and computational biomechanics for accurate implant fit calculations and generates interactive 3D surgical plans.
- The FDA clearance allows Formus Labs to enter the US market with its advanced surgical planning solution.
Main AI News:
Formus Labs, a prominent New Zealand-based healthcare technology company, has recently obtained the highly sought-after 510(k) clearance from the United States Food and Drug Administration (FDA) for its state-of-the-art automated radiological image processing software.
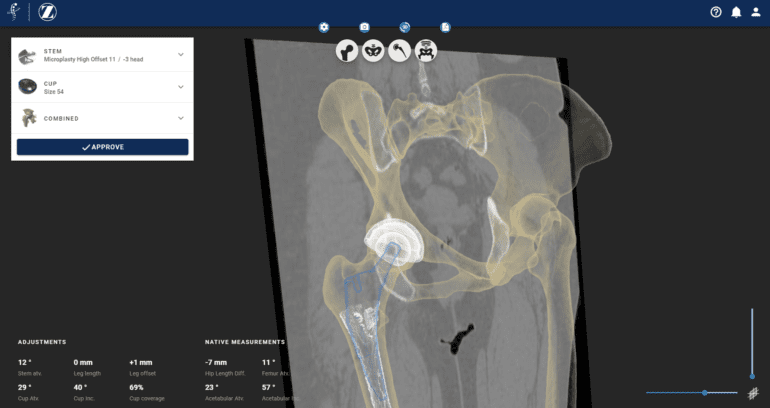
Designed specifically for pre-operative planning of hip replacement surgeries, the groundbreaking software, known as Formus Hip, incorporates cutting-edge AI algorithms and computational biomechanics to deliver accurate assessments of implant fit and create fully interactive 3D surgical plans that are both actionable and easily understandable.
The FDA’s rigorous clearance process serves as a testament to the advanced capabilities and reliability of Formus Hip. With this important regulatory milestone achieved, Formus Labs is now poised to make significant strides in the highly competitive US market, where the demand for innovative and efficient surgical planning solutions remains high. Leveraging its groundbreaking technology, Formus Labs is well-positioned to address the complex challenges faced by surgeons and enhance the precision and success rates of hip replacement procedures.
Formus Hip’s AI-powered capabilities enable it to swiftly process and analyze radiological images, providing comprehensive insights and recommendations to surgeons. By accurately calculating the ideal fit for each patient’s implant, the software empowers surgeons with critical information to make informed decisions and optimize surgical outcomes. The interactive 3D surgical plans generated by Formus Hip allow surgeons to visualize the procedure in great detail, facilitating effective communication with the surgical team and enhancing overall surgical efficiency.
The FDA clearance marks a significant milestone for Formus Labs, validating the company’s commitment to developing innovative healthcare solutions that improve patient care and surgical outcomes. With its groundbreaking technology and FDA approval in hand, Formus Labs is well-positioned to revolutionize the field of hip replacement surgeries and provide surgeons with a powerful tool for precise pre-operative planning.
As Formus Labs prepares to fully enter the US market, healthcare professionals and patients alike can look forward to the transformative benefits offered by Formus Hip. By combining the power of AI and computational biomechanics, this advanced software sets a new standard in hip surgery planning, ultimately leading to improved patient outcomes and enhanced quality of life.
Conlcusion:
Formus Labs’ achievement of FDA 510(k) clearance for its advanced hip surgery planning software signifies a significant development in the market. This regulatory approval not only validates the effectiveness and reliability of Formus Hip but also opens up opportunities for the company to tap into the lucrative US market. With its AI-powered capabilities and ability to deliver precise and interactive surgical plans, Formus Labs is poised to revolutionize the field of hip replacement surgeries.
This breakthrough solution has the potential to enhance surgical outcomes, improve patient care, and shape the future of surgical planning technologies. As Formus Labs enters the US market, it is likely to disrupt the existing landscape, creating new possibilities for surgeons and patients alike and driving further advancements in the field of orthopedic surgery.