TL;DR:
- Scientists at Baidu Research have developed an AI tool that optimizes gene sequences in mRNA vaccines, improving their stability and potency.
- The tool, called LinearDesign, designs mRNA sequences with intricate shapes and structures, resulting in more stable delivery to cells and increased production of antigens.
- LinearDesign has shown remarkable results, inducing antibody responses up to 128 times greater than conventional vaccines in mouse studies.
- The tool has the potential to eliminate the need for ultracold storage facilities, expanding vaccine distribution to resource-limited regions.
- LinearDesign has been used to optimize the SW-BIC-213 COVID-19 vaccine and is being licensed by Sanofi for their experimental mRNA products.
- Other studies have explored alternative algorithms and manual fine-tuning for further optimization.
- The tool’s safety is being monitored in human trials, with initial results showing comparable side effects to other mRNA-based COVID-19 vaccines.
- Ongoing research and evaluation are essential to understand potential immune reactions and ensure the development of safe and effective vaccines.
Main AI News:
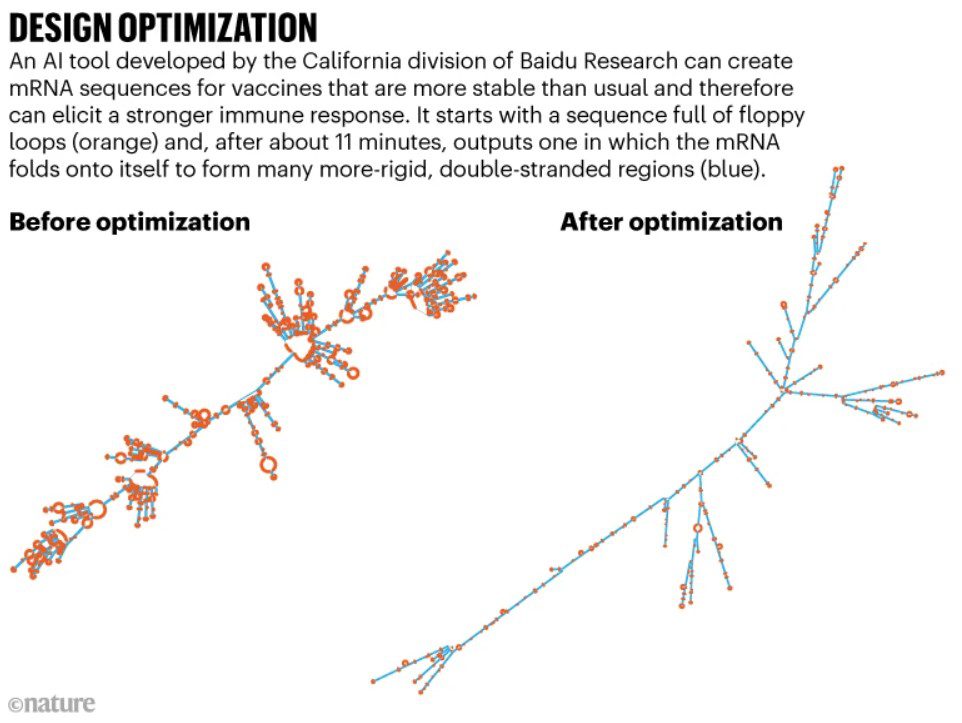
An innovative breakthrough has emerged in the field of mRNA vaccines, offering tremendous potential for global deployment. Scientists at Baidu Research, the California division of an esteemed AI company based in Beijing, have developed a remarkable AI tool that optimizes gene sequences within mRNA vaccines. By harnessing techniques from computational linguistics, this cutting-edge software designs mRNA sequences with intricate shapes and structures, surpassing the current standards.
As a result, the genetic material endures for extended periods, enabling the delivery of more stable mRNA to the recipient’s cells. This heightened stability prompts the protein-making machinery within the body to produce a greater number of antigens. Consequently, immunized individuals gain a significant boost in protective antibodies, bolstering their ability to combat infectious diseases effectively.
Moreover, the advanced structural complexity of the optimized mRNA provides a substantial improvement in safeguarding the vaccine against degradation. Notably, during the tumultuous COVID-19 pandemic, mRNA-based shots targeting the SARS-CoV-2 coronavirus required transportation and storage at temperatures below -15°C to maintain their stability.
Unfortunately, this posed a significant challenge in resource-limited regions where ultracold storage facilities were inaccessible. However, with the aid of AI-driven optimization, a more resilient mRNA vaccine product could eliminate the dependence on cold-chain equipment for handling such vaccines.
Experts within the field are captivated by this breakthrough, recognizing its unprecedented significance. Dave Mauger, a computational RNA biologist who formerly worked at Moderna, a renowned mRNA vaccine manufacturer based in Cambridge, Massachusetts, describes the new methodology as “remarkable.” He emphasizes that the computational efficiency of the AI tool surpasses anything previously witnessed, marking a significant milestone in this cutting-edge domain.
In the realm of vaccine development, mRNA sequences are already modified by researchers to align with the genetic preferences of cells, a process known as codon optimization. This optimization enhances the efficiency of protein production. However, the groundbreaking LinearDesign tool developed by Baidu goes beyond codon optimization, introducing a novel approach that results in the formation of double-stranded segments within the typically single-stranded mRNA molecule. These double-stranded segments provide increased rigidity, further enhancing the vaccine’s efficacy and stability.
Impressively, LinearDesign is capable of running on a desktop computer and completes its optimization process within minutes. Validation tests have demonstrated remarkable outcomes, with vaccines optimized by LinearDesign inducing antibody responses in mice up to 128 times greater than those achieved by conventional codon-optimized vaccines. Furthermore, the algorithm has successfully extended the shelf stability of vaccine designs up to six times in standard test-tube assays conducted at body temperature.
The transformative impact of LinearDesign has garnered high praise from experts in the field. Yujian Zhang, former head of mRNA technology at StemiRNA Therapeutics in Shanghai, China, who led the experimental-validation studies, describes the tool as a “tremendous improvement.” Zhang and his colleagues have thus far tested LinearDesign-enhanced vaccines against COVID-19 and shingles in mice.
However, the versatility of this technique is expected to extend to the development of mRNA vaccines for a wide range of diseases. Liang Huang, the visionary computational biologist at Oregon State University in Corvallis, who spearheaded the creation of the LinearDesign tool during his time at Baidu, emphasizes its potential applications in mRNA-based therapeutics as well.
These groundbreaking findings were published on 2 May in the esteemed scientific journal, Nature. The practical application of the LinearDesign tool has already yielded promising results in vaccine optimization. One notable example is the optimization of the SW-BIC-213 COVID-19 vaccine developed by StemiRNA, which received emergency use approval in Laos last year.
Furthermore, a licensing agreement established in 2021 has enabled the French pharmaceutical giant Sanofi to utilize LinearDesign for its own experimental mRNA products. Executives from both StemiRNA and Sanofi emphasize that vaccine performance is influenced by various design factors, and while LinearDesign is one algorithm among many, it undeniably contributes to the optimization process.
Another noteworthy research study conducted by Rhiju Das and his team at Stanford School of Medicine showcased the potential for even greater protein expression from mRNA by removing specific loop patterns from the strands. This discovery highlights the possibility of alternative algorithms being preferred for optimization, as suggested by theoretical chemist Hannah Wayment-Steele, formerly part of Das’s team and now associated with Brandeis University. Alternatively, manual fine-tuning of mRNA sequences optimized by LinearDesign may lead to further improvements in vaccine performance.
Nevertheless, David Mathews, a computational RNA biologist at the University of Rochester Medical Center and co-founder of Coderna.ai alongside Liang Huang, emphasizes the significant contribution of LinearDesign. According to Mathews, the algorithm serves as a valuable starting point for optimization, enabling researchers to enter the realm of vaccine development with a solid foundation.
Mathews and Huang are currently focused on advancing the software through their start-up, Coderna.ai, based in Sunnyvale, California. Their initial task involves updating the platform to accommodate the chemical modifications commonly found in approved and experimental mRNA vaccines. It is worth noting that the current version of LinearDesign is based on an unmodified mRNA platform that has fallen out of favor among most vaccine developers.
As researchers delve deeper into the intricacies of mRNA vaccine optimization, a combination of advanced algorithms, manual fine-tuning, and incorporation of chemical modifications is expected to yield even more optimal solutions. The collaboration between academia, pharmaceutical companies, and AI-driven technologies brings us closer to the development of highly effective and stable mRNA vaccines, opening new possibilities for disease prevention and control.
While mouse studies and cell experiments provide valuable insights, it is crucial to consider the transition to human trials. Researchers are aware that the immune system has evolved to identify certain RNA structures as foreign, particularly the twisted ladder shapes commonly found in viruses that encode their genomes using double-stranded RNA. Consequently, there is concern among some researchers that optimization algorithms like LinearDesign could potentially generate vaccine sequences that trigger harmful immune reactions in humans.
Anna Blakney, an RNA bioengineer at the University of British Columbia, raises this concern, emphasizing the potential liability associated with such a scenario. However, the initial results from human clinical trials involving StemiRNA’s SW-BIC-213 vaccine indicate that the additional structural complexity introduced by LinearDesign has not posed any significant problems. In the small booster trials conducted thus far, the vaccine’s side effects have been comparable to those reported with other mRNA-based COVID-19 vaccines. Nevertheless, Blakney highlights the need for ongoing monitoring and research to gain a deeper understanding of potential immune reactions over the coming years.
The transition from preclinical studies to human trials is a critical phase in vaccine development. It requires careful evaluation of safety and efficacy to ensure the well-being of vaccine recipients. As the scientific community continues to explore the optimization of mRNA vaccines, it remains essential to address any concerns regarding immune reactions and strive for comprehensive understanding and continuous improvement in the pursuit of safe and effective vaccines.
Source: Adapted from Ref. 1
Conlcusion:
The development of the AI-driven optimization tool for mRNA vaccines represents a significant advancement in the market. This technology offers the potential to enhance the potency and stability of vaccines, revolutionizing the field of immunization. The optimization tool’s ability to design mRNA sequences with intricate shapes and structures, resulting in increased stability and antigen production, holds promise for improved vaccine efficacy. Additionally, the tool’s potential to eliminate the dependency on ultracold storage facilities opens up new market opportunities, allowing for broader distribution of vaccines to resource-limited regions.
The collaborations between academia, pharmaceutical companies, and AI-driven technologies further accelerate innovation in vaccine development. While ongoing monitoring of immune reactions is necessary, this advancement paves the way for the future of mRNA vaccines, presenting lucrative prospects in the global healthcare market.