- Dr. Ori Avinoam and Dr. Assaf Zaritsky collaborated to develop a machine learning model.
- The model tracked muscle fiber development from stem cells, revealing a novel regulatory checkpoint.
- Cellular differentiation was found to be a decentralized process, challenging prior assumptions.
- Inhibiting the enzyme p38 stalled cells at the fusion stage, unveiling its role as a crucial checkpoint.
- Molecular analysis indicated heightened cytoskeletal protein expression in inhibited cells, elucidating the underlying mechanism.
Main AI News:
In the digital era of life sciences, the synergy between biology and computational science has opened unprecedented avenues for exploration. Researchers, armed with vast datasets, now leverage sophisticated computational models to unravel the intricacies of biological phenomena.
Dr. Ori Avinoam, hailing from the Biomolecular Sciences Department at the Weizmann Institute of Science, embarked on a quest to demystify a longstanding biological puzzle: the genesis of new muscle fibers from stem cells. Teaming up with Dr. Assaf Zaritsky from the Software and Information Systems Engineering Department at Ben-Gurion University of the Negev, they pioneered a machine learning model to decipher this enigmatic process.
Their breakthrough, detailed in Molecular Systems Biology, unveiled a groundbreaking regulatory checkpoint governing muscle tissue development. Stem cells, latent in adult muscles, spring into action during growth, exertion, or injury. The journey begins with cellular division, followed by differentiation, wherein cells acquire specialized traits essential for muscle function.
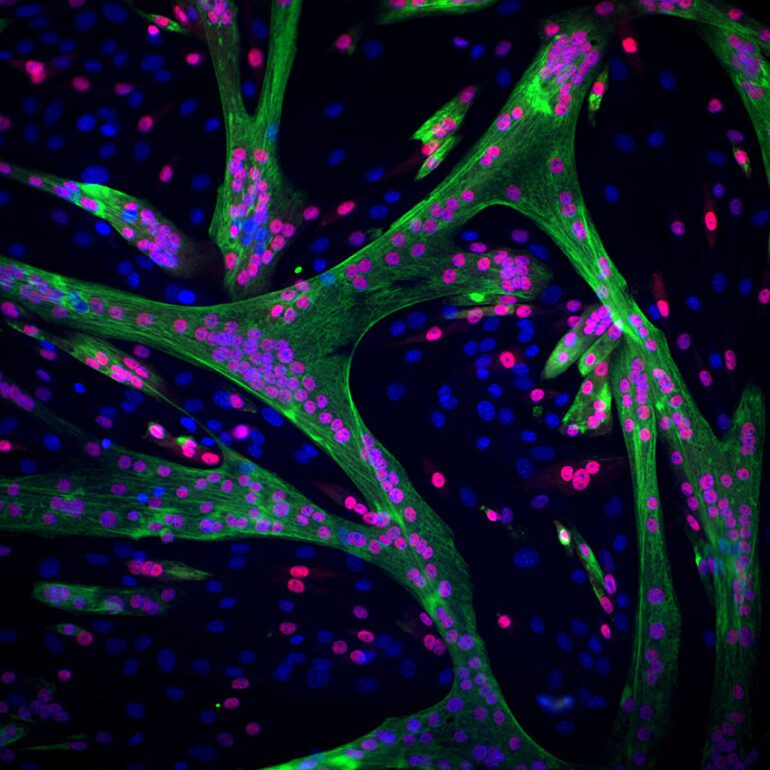
Tracking this intricate dance of cellular transformation, researchers Giulia Zarfati and Adi Hazak meticulously documented muscle fiber development in real-time. Leveraging fluorescent labeling, they monitored cellular movement and protein fiber synthesis—a hallmark of muscle maturation.
Collaborating with research student Amit Shakarchy, the interdisciplinary team bridged the gap between biology and machine learning. Through meticulous integration of temporal data, they crafted a model that delineated the dynamic progression of muscle differentiation.
Their model unveiled a nuanced narrative of cellular evolution, challenging conventional wisdom with its revelation of decentralized differentiation patterns. Moreover, it illuminated a pivotal checkpoint preceding cellular fusion into muscle fibers.
By inhibiting the activity of the enzyme p38, implicated in muscle development, researchers uncovered critical insights. While cells completed differentiation sans the enzyme, they faltered at the fusion stage, underscoring the enzyme’s role as a gatekeeper of muscle maturation.
Further analysis illuminated the molecular intricacies underlying this checkpoint. Inhibited cells exhibited heightened expression of cytoskeletal proteins, priming them for fusion yet stalling at the cusp of completion.
Dr. Avinoam emphasizes the broader implications of their findings, suggesting a paradigm shift in disease monitoring. The model’s ability to track cellular dynamics in real-time heralds a new era in disease surveillance, promising unparalleled insights into dynamic biological processes.
Conclusion:
This breakthrough in understanding muscle development not only sheds light on fundamental biological processes but also opens doors for potential medical applications. The ability to track cellular dynamics in real-time could revolutionize disease monitoring and intervention strategies, paving the way for personalized healthcare solutions tailored to individual needs. Businesses in the biotech and pharmaceutical sectors should take note of these advancements, as they may inform the development of novel therapies and diagnostic tools in the near future.