TL;DR:
- Microbes play crucial roles in elemental cycles, plant growth, and disease development in ecosystems.
- Research efforts expand microbial DNA sequence databases but lack comprehensive biological information about proteins.
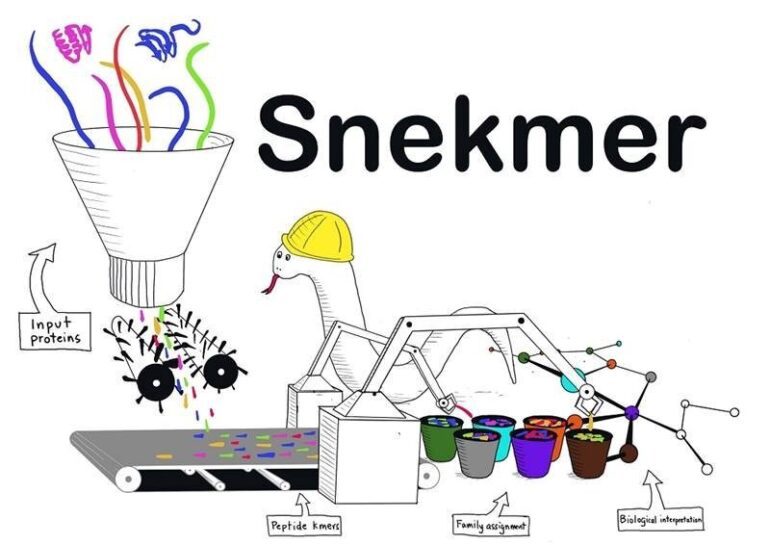
- Snekmer, a machine learning-based program, predicts protein function by modeling protein families quickly.
- It aids in engineering microbes for sustainable bioenergy and bioproducts.
- Snekmer integrates into high-performance computing environments and the DOE KBase framework.
- It facilitates genome and metagenome sequence annotation and enables the modeling of engineered microbe effects.
- Snekmer studies microbial evolution and microbiome patterns.
- Traditional methods struggle to predict protein function for a significant portion of bacterial protein sequences.
- Snekmer reduces sequence space and employs machine learning to generate accurate protein family models.
- It addresses the need for improved protein function prediction and classification.
Main AI News:
In the world of microbiology, the role of microbes in driving fundamental processes of life on Earth cannot be overstated. These minuscule organisms exert their influence on global elemental cycles, orchestrating the intricate movement of carbon, nitrogen, and various other essential elements.
Moreover, they possess the power to promote plant growth or catalyze the development of diseases, making them indispensable in every ecosystem. As research endeavors continue to expand the ever-growing database of microbial DNA sequences, scientists strive to unlock the wealth of biological information encoded within these sequences, particularly pertaining to proteins.
Proteins, the workhorses of molecular machinery, hold the key to unlocking the potential of engineered microbes for sustainable bioenergy and other valuable bioproducts. To harness this potential, scientists must gain a comprehensive understanding of protein function and the intricate mechanisms that govern it. Traditionally, protein function inference has relied on comparing newly discovered proteins with reference databases of already characterized proteins. However, this approach proves to be arduous and impractical when dealing with massive databases, presenting a significant hurdle in the quest for knowledge.
Enter the groundbreaking solution that has captivated the scientific community: Snekmer. By harnessing the power of machine learning, Snekmer propels protein function prediction into a new era of efficiency and scalability. Developed by a collaborative team of researchers from the Pacific Northwest National Laboratory, Baylor University, and Oregon Health & Science University, this cutting-edge program enables scientists to swiftly model entire families of proteins, empowering them with a deeper understanding of their functionalities.
The application of Snekmer extends beyond its revolutionary impact on protein research. With its seamless integration into high-performance computing environments, Snekmer emerges as a user-friendly tool that scientists can readily deploy for their investigations. Notably, it has been seamlessly incorporated into the DOE KBase framework as a new application, facilitating the annotation of genome and metagenome sequences. This breakthrough promises to revolutionize the study of biological protein molecules in microbes, paving the way for novel applications in engineered microbe technologies.
By equipping scientists with the ability to annotate genomes and metagenomes, Snekmer enables a more nuanced exploration of the effects of engineered microbes. This encompasses their influence on climate dynamics, their role in bolstering crop health, and their potential for enhancing bioproduction processes. Additionally, Snekmer serves as an invaluable tool for studying the evolution of microbes and deciphering intricate patterns within microbiomes, offering scientists a comprehensive view of these complex systems.
The limitations of current methods for predicting protein function loom large, hindering progress in understanding intricate systems like soil microbiomes. Traditional protocols heavily rely on pair-wise alignments, which have become computationally intractable and increasingly challenging to interpret as databases continue to expand.
Furthermore, alignment-based models heavily depend on initial training sets, posing a risk of obsolescence as new sequence diversity is discovered. Compounding this challenge is the fact that many bacterial proteins remain functionally unassigned or are solely attributed with a generic function based solely on taxonomic understanding.
Addressing this pressing need for a transformative approach, the scientific minds at Pacific Northwest National Laboratory, Baylor University, and Oregon Health & Science University have birthed Snekmer—a software tool that harnesses the inherent redundancy of amino acid residue properties to shrink the sequence space. By utilizing concise protein sequence features known as “kmers” and employing machine learning algorithms, Snekmer generates robust protein family models with unparalleled accuracy.
Snekmer empowers users to recode protein sequences into reduced alphabet kmer vectors, laying the groundwork for constructing supervised classification models. These models, trained on input protein families, enable precise and efficient protein functional classification. With Snekmer, scientists are equipped with a versatile tool that unlocks the full potential of protein function prediction, propelling research into uncharted territories.
Conclusion:
Snekmer’s revolutionary capabilities in predicting protein function through machine learning have immense implications for the market. It unlocks new possibilities in the field of engineered microbes, particularly in sustainable bioenergy and bioproducts. Its integration into high-performance computing environments and the DOE KBase framework positions it as a valuable tool for genome and metagenome sequence annotation.
Additionally, Snekmer’s ability to study microbial evolution and microbiome patterns opens up avenues for further research and applications. With traditional methods struggling to keep up with expanding databases, Snekmer’s reduction of sequence space and accurate protein family models offer a more efficient and reliable solution. As a result, Snekmer is poised to drive advancements in the understanding and utilization of proteins, fostering innovation and progress in various industries.