TL;DR:
- MIT and Takeda collaborate to enhance pharmaceutical manufacturing processes using physics and machine learning.
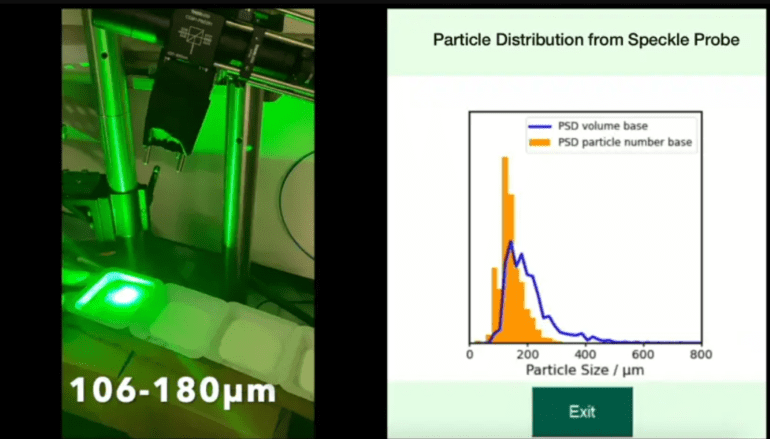
- Their innovative approach, known as PEACE, categorizes rough particle surfaces in pills and powders.
- The goal is to increase efficiency, accuracy and reduce failed batches of products.
- By illuminating particles with a laser and applying physics-based equations, the team can analyze particle sizes.
- The machine learning algorithm benefits from the physics-based approach, requiring minimal training data.
- This breakthrough could lead to more efficient, sustainable, and cost-effective drug production.
- The PEACE mechanism offers real-time monitoring of particle size distribution, a significant advancement for the industry.
- The collaboration between MIT and Takeda demonstrates the successful integration of academia and industry.
- Potential applications extend beyond pharmaceutical manufacturing, and the team has already filed for patents.
Main AI News:
In the realm of medicine, precision and efficiency are paramount. That’s why a team of engineers and researchers from the Massachusetts Institute of Technology (MIT) and Takeda Pharmaceutical Company Limited have joined forces to revolutionize pharmaceutical manufacturing processes using the power of physics and machine learning. Their groundbreaking collaboration aims to enhance the production of pharmaceutical pills and powders, leading to increased efficiency, accuracy, and fewer failed batches of products.
The traditional process of manufacturing pills and tablets requires isolating the active pharmaceutical ingredient from a suspension and drying it. This delicate procedure relies heavily on the observation skills of human operators, who monitor industrial dryers, agitate the material, and ensure it reaches the desired state for compression into medicine. However, the subjective nature of this task presents challenges.
Enter the research team from MIT and Takeda, who have published a recent Nature Communications paper introducing a novel approach to this longstanding problem. By combining physics and machine learning, they have developed a method to categorize the rough surfaces that characterize particles in pharmaceutical mixtures. This groundbreaking technique, known as the physics-enhanced autocorrelation-based estimator (PEACE), has the potential to transform pharmaceutical manufacturing processes, significantly improving efficiency, accuracy and minimizing the occurrence of failed batches.
Professor Allan Myerson, a renowned figure in the field and one of the authors of the study, emphasizes the importance of reliability, time reduction, and compliance in pharmaceutical manufacturing. He states, “Failed batches or failed steps in the pharmaceutical process are very serious. Anything that improves the reliability of pharmaceutical manufacturing, reduces time, and improves compliance is a big deal.”
This pioneering work is part of an ongoing collaboration between Takeda and MIT, initiated in 2020 under the MIT-Takeda Program. This strategic partnership leverages the extensive expertise of both institutions to tackle challenges at the intersection of medicine, artificial intelligence, and healthcare.
Conventionally, determining the adequacy of mixing and drying compounds involves halting industrial-sized dryers and taking samples off the manufacturing line for testing. Takeda researchers recognized the potential of artificial intelligence in streamlining this process and reducing production stoppages. Initially, they aimed to employ videos to train a computer model to replace human operators. However, the subjectivity in selecting training videos posed a significant hurdle. To overcome this, the MIT-Takeda team devised a new approach that involves illuminating particles with a laser during filtration and drying, coupled with physics and machine learning-based analysis of particle size distribution.
Qihang Zhang, the first author of the study and a doctoral student in MIT’s Department of Electrical Engineering and Computer Science, explains the methodology, stating, “We just shine a laser beam on top of this drying surface and observe.” This laser interaction with the mixture is described by a physics-derived equation, while machine learning algorithms characterize particle sizes. Notably, this process does not require frequent starting and stopping, resulting in a more secure and efficient workflow compared to standard operating procedures.
Furthermore, the machine learning algorithm exhibits remarkable efficiency in learning its task, thanks to the utilization of physics to compensate for the limited training data. Zhang adds, “We utilize the physics to compensate for the lack of training data, so that we can train the neural network in an efficient way. Only a tiny amount of experimental data is enough to get a good result.”
Currently, the pharmaceutical industry lacks inline processes for measuring particles within a powder during mixing, except for slurry products where crystals float in a liquid. However, when liquids are filtered and dried, their composition changes, necessitating new measurements. The utilization of the PEACE mechanism not only accelerates and streamlines the process but also ensures safer operations by minimizing the handling of highly potent materials.
The potential implications for pharmaceutical manufacturing are significant, promising enhanced efficiency, sustainability, and cost-effectiveness by reducing the need for extensive experimentation during product development. Charles Papageorgiou, director of Takeda’s Process Chemistry Development group and one of the study’s authors, acknowledges the long-standing industry challenge of monitoring drying mixtures’ characteristics in real-time. He states, “It is a problem that a lot of people are trying to solve, and there isn’t a good sensor out there. This is a pretty big step change, I think, with respect to being able to monitor, in real-time, particle size distribution.”
Papageorgiou suggests that this innovative mechanism could find applications in other industrial pharmaceutical operations as well. In the future, laser technology may evolve to incorporate video imaging, enabling manufacturers to employ cameras for analysis instead of laser measurements. Currently, Takeda is assessing the tool’s performance with different compounds in their laboratory.
These groundbreaking results are a direct outcome of the collaboration between Takeda and three departments at MIT: Mechanical Engineering, Chemical Engineering, Electrical Engineering and Computer Science. Over the past three years, these esteemed institutions have collaborated on 19 projects, harnessing the power of machine learning and artificial intelligence to address healthcare and medical industry challenges through the MIT-Takeda Program.
Typically, it takes years for academic research to translate into industrial processes. However, this direct collaboration between MIT and Takeda holds promise for expediting this timeline. The proximity of Takeda’s facilities to MIT’s campus has allowed researchers to set up experiments in the company’s lab and receive real-time feedback, enabling MIT researchers to align their work with Takeda’s equipment and operations.
Conclusion:
The collaboration between MIT and Takeda, leveraging the power of physics and machine learning, has the potential to revolutionize the pharmaceutical manufacturing market. The introduction of the PEACE mechanism allows for increased efficiency, accuracy and reduced failed batches of products. This breakthrough offers real-time monitoring and characterization of particles, addressing a long-standing industry challenge. Furthermore, the successful integration of academia and industry in this collaboration sets a precedent for future advancements in the healthcare and medical sectors. The implications extend beyond pharmaceuticals, opening doors for innovation in other industrial operations. Overall, this breakthrough signifies a significant step forward in optimizing manufacturing processes, enhancing competitiveness, and driving cost-effectiveness in the pharmaceutical market.