TL;DR:
- The COVID Moonshot initiative began as a spontaneous collaboration in March 2020.
- Over 200 scientists from academia and biopharma crowdsourced new antiviral molecules.
- Lead candidate for COVID-19 treatment now in pre-clinical evaluation.
- Consortium dedicated to patent-free, affordable antiviral drugs for pandemics.
- The innovative approach leveraged crowdsourcing, machine learning, and high-throughput screening.
- Valuable open-source data shared, accelerating global research.
- Impactful discoveries, including Shionogi’s Xocova, facilitated by open science.
- The transformative potential of crowd-sourcing is highlighted.
- The initiative continues as the ASAP discovery consortium for pandemic preparedness.
Main AI News:
In a world grappling with the challenges of a global pandemic, the COVID Moonshot initiative emerged as a beacon of hope and innovation. What began as a spontaneous virtual collaboration in March 2020 in response to a Twitter appeal has evolved into a remarkable journey of scientific discovery. A diverse group of over 200 scientists and students from academia and biopharma united in a race against time, driven by a singular goal: to identify new molecules capable of combating the formidable SARS-CoV-2 virus.
This unprecedented endeavor, marked by its crowdsourced and fully open nature, achieved the remarkable feat of rapidly identifying and developing novel compounds with exceptional antiviral properties against a crucial enzyme of the SARS-COV-2 virus—the main protease (Mpro). Today, the lead candidate resulting from this groundbreaking effort is undergoing pre-clinical evaluation in collaboration with the Drugs for Neglected Disease initiative (DNDi).
The COVID Moonshot is committed to pioneering the discovery of safe, globally affordable antiviral drugs not only for COVID-19 but also for future viral pandemics. It champions a direct-to-generic, patent-free approach, breaking away from traditional pharmaceutical paradigms.
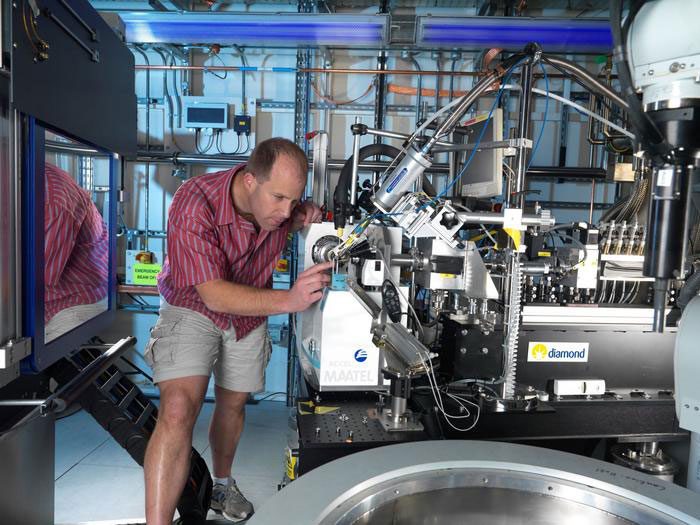
The consortium’s recent publication sheds light on their discovery of a non-covalent, non-peptidic inhibitor scaffold exhibiting lead-like properties, setting it apart from current main protease inhibitors. Their innovative approach harnessed the power of crowdsourcing, machine learning, exascale molecular simulations, and high-throughput structural biology and chemistry. This endeavor was fortified by data from a large-scale experiment conducted at the onset of the pandemic at Diamond Light Source’s XChem facility for crystallographic fragment screening. In just weeks, 1,495 fragment-soaked crystals were screened, leading to the identification of 78 hits that densely populated the enzyme’s active site.
The team’s achievements extend beyond the identification of promising compounds. They also generated a comprehensive map of the structural plasticity of the SARS-CoV-2 main protease, unraveling extensive structure-activity relationships for multiple chemotypes and providing a wealth of biochemical activity data. Notably, all compound designs (exceeding 18,000), crystallographic data (comprising over 840 ligand-bound X-ray structures), assay data (exceeding 10,000 measurements), and synthesized molecules (over 2,400 compounds) were swiftly and openly shared. This wealth of information forms a valuable, open, and IP-free knowledge base for future anti-coronavirus drug discovery.
The consortium’s commitment to open science is resolute. By making their data immediately accessible, they aim to accelerate global research efforts, enabling other scientists to build upon their foundation. Dr. Lizbe Koekemoer, one of the lead authors and a team leader at the Centre for Medicines Discovery, University of Oxford, emphasizes the uniqueness of this resource: “The data set enclosed in the Science publication provides a unique resource linking comprehensive structural data, fragment hits, multiple chemical scaffolds, as well as biochemical and cellular assay data that can be viewed and exploited by other scientists.”
Moreover, this endeavor represents a milestone in the world of drug discovery. Dr. Daren Fearon, co-lead author and Senior Beamline Scientist at Diamond Light Source underscores the magnitude of this achievement: “This is the first time such a large number of protein-ligand structures have been generated for a drug discovery campaign and released in the public domain. It is a testament to Diamond’s high-throughput crystallography infrastructure, but also the astonishing coordination across many research groups worldwide under enormous pressure.”
The impact of open science is vividly illustrated by the case of the Shionogi clinical candidate S-217622, now available in Japan under emergency approval as Xocova [ensitrelvir]. This compound’s discovery was facilitated by the data generated at Diamond and openly shared with the scientific community.
Professor Frank von Delft, Principal Beamline Scientist at Diamond, Professor for Structural Chemical Biology at the University of Oxford, and one of the founders of the consortium, comments on the transformative power of open science: “Open science efforts have transformed many areas of biosciences. The COVID Moonshot provides an exemplar of a viable route to open science early drug discovery leading to advances in infectious diseases drug discovery—a research area of grave public importance but one which is chronically underfunded by the private sector. The Moonshot structure-enabled drug discovery campaign targeting the coronavirus main protease is providing a roadmap for the potential development of future antivirals.”
In essence, this publication underscores the immense value that crowd-sourcing can bring to drug discovery. The COVID Moonshot project, with its collaborative approach and unwavering commitment to open science, stands as a testament to the potential of collaboration as a catalyst for innovation.
Dr. Annette von Delft, University of Oxford, summarizes it aptly: “This publication showcases the enormous value that crowd-sourcing can bring to drug discovery. The COVID Moonshot project has been unique in its collaborative approach and commitment to open science and demonstrates how collaboration can be a driver for innovation.”
As the COVID Moonshot’s legacy continues, it now assumes a new identity—the ASAP discovery consortium. Standing for an AI-driven Structure-enabled Antiviral Platform, this consortium is dedicated to the discovery and development of novel broad-spectrum small molecule inhibitors against coronaviruses, flaviviruses, and enteroviruses, with a focus on pandemic preparedness. This initiative is the result of collaborative efforts from the Nuffield Department of Medicine at the University of Oxford, Diamond Light Source, PostEra, Weizmann Institute of Science, MedChemica Ltd, Icahn School of Medicine at Mount Sinai, Enamine Ltd, Memorial Sloan Kettering Cancer Center, and Thames Pharma Partners LCC.
Conclusion:
The COVID Moonshot Consortium’s journey exemplifies the power of collaboration, open science, and innovation in the face of global challenges. Their pioneering efforts have not only advanced our understanding of antiviral drug discovery but also set a precedent for future endeavors in infectious disease research.